U.S. Abortion Pill Study Finds Alarming Rate of Harm for Women
A new study from the United States, titled The Abortion Pill Harms Women: Insurance Data Reveals One in Ten Patients Experiences a Serious Adverse Event, brings to light the lack of safety of the abortion pill for women. While we already knew the harm to pre-born children, the statistics revealed by this study show a much higher rate of harm to women than that reported by the drug company on information given with the abortion pill.
Conducted by the Ethics and Public Policy Center, this study is the largest-known study of the abortion pill to date. Researchers evaluated 865,727 abortions through prescriptions of mifepristone from 2017 to 2023, as reported in insurance records. Almost 11% of all uses of Mifeprex, the abortion pill used in the United States, resulted in some sort of serious adverse event within 45 days of the abortion. Nearly five percent of cases required a visit to the emergency room and nearly three percent required a surgical abortion when the medical abortion failed. Hemorrhaging (3.3%), infection (1.3%), and sepsis (0.1%) were the most common conditions.
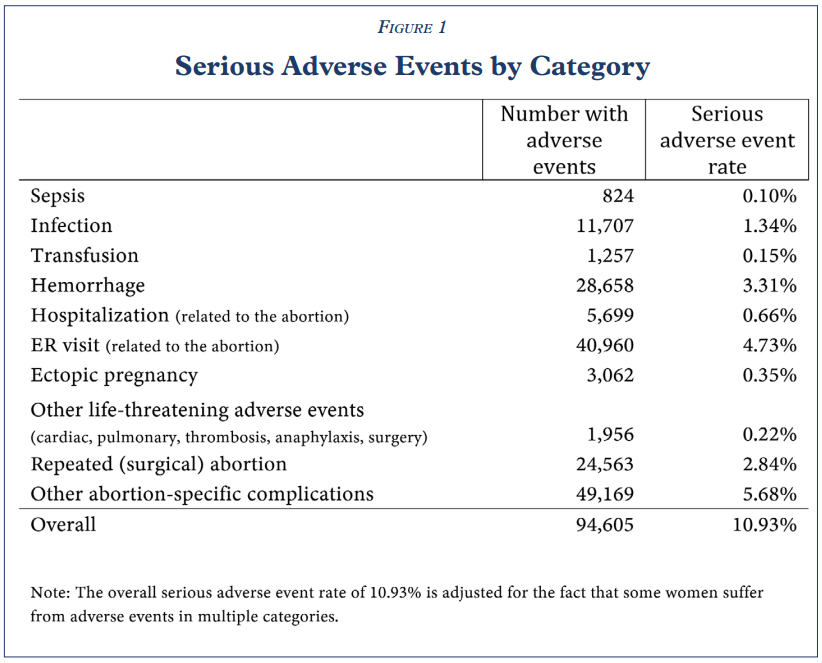
Based on these findings, the researchers found that “mifepristone, as used in real-world conditions, is not ‘safe and effective’” and requires additional patient safety protocols. While the Federal Food and Drug Administration (FDA) in 2000 found a rate of adverse events that was below 0.5% during the drug’s approval process, the clinic studies used during the approval used “ideal world” conditions: healthy women, a carefully controlled regimen, and strict inclusion criteria. The “real world” application of the abortion pill – where women can self-report how far along they are in pregnancy and take the pills at home without physician oversight, for example – leads to a 28-fold increase in the number adverse effects.
The Abortion Pill in Canada
How does this compare to Canada?
Health Canada approved Mifegymiso, commonly referred to as the abortion bill, in 2015, fifteen years after a similar drug was approved in the United States. Initially, Health Canada authorized Mifegymiso for medical abortions up to 7 weeks (as measured from the first day of a woman’s last period), provided that physicians do the following:
- Ensure that patients have access to emergency medical care in the 14 days following administration of mifepristone.
- Schedule follow-up appointment 7 to 14 days after patients take mifepristone to confirm complete pregnancy termination.
- Exclude ectopic pregnancy and confirm gestational age by ultrasound.
- Counsel each patient on the risks and benefits of Mifegymiso, including bleeding, infection, and incomplete abortion.
- Obtain the patient’s written informed consent to take the drug.
- Complete the mandatory Mifegymiso education and registration programs.
As a part of their approval process, Health Canada considered the benefits of Mifegymiso outweighed the risks of the drug. The “benefit” of the abortifacient is the termination of the pregnancy.
The risks of using the abortion pill are far more numerous. The three pivotal studies used in Health Canada’s approval process found that treatment failure (e.g. patient requiring a surgical intervention for any reason, which may include a viable pregnancy, a non-viable persistent pregnancy, persistent bleeding or abdominal pain) occurred in 2%-4.8% of women, a rate far higher than the American FDA’s records. Other serious safety concerns included infection and sepsis, heavy bleeding, and the embryotoxicity of Mifegymiso for a failed abortion or a future pregnancy. One of the studies found that women who took Mifegymiso experienced the following adverse effects: pain (92%), nausea (68%), weakness (56%), dizziness (45%), headache (42%), fever (39%), diarrhea (36%), and vomiting (29%).
Compare this to the Ethics & Public Policy Center study’s findings. (One of the studies submitted for consideration by Health Canada stated that Mifegymiso was bioequivalent to the Mifeprex used in the United States, so the two drugs at issue here, although different, are very similar.) The Ethics & Public Policy Center study’s findings are based on 865,727 cases, almost 1000 times more than Health Canada’s 934 cases, and it finds the risks of the abortion pill to be 2-4 times higher than Health Canada’s findings from their small sample.
Despite the known risks, the safeguards around the abortion pill were relaxed mere months after its introduction. Mifegymiso was first rolled out in Canada in January 2017 but, by May of the same year, Health Canada removed the requirement for physicians to take specialized training before prescribing the drug, allowed pharmacists to prescribe the drug, approved use for pregnancies up to 9 weeks gestation, and removed the requirement for an ultrasound. The current product monograph describing the safety of Mifegymiso has not been updated since 2019.
| ORIGINAL SAFEGUARDS | CURRENT SAFEGUARDS |
| Restricted past 7 weeks | |
| Restricted to physicians prescribing | |
| Ensure access to emergency medical care | Ensure access to emergency medical care |
| Follow-up appointment with a physician | Follow-up appointment with a physician |
| Exclude possibility of ectopic pregnancy and confirm gestational age by ultrasound | |
| Counsel patient on risks of Mifegymiso | Counsel patient on risks of Mifegymiso |
| Obtain written consent to take drug | Obtain written consent to take drug |
| Complete education program |
Pro-life is pro-woman
Over 40% of all reported abortions in Canada are done through the abortion pill. Abortion advocates have pushed for these relaxed safeguards, but this study is evidence once again that safeguards matter. Insisting on availability over safety shows a disregard for the lives of women, a disregard that makes sense when it comes from the same people who are happy to disregard the lives of pre-born children.
As pro-life Canadians, we show our true valuing of life when we speak for the safety of both women and children. The abortion pill isn’t safe – it ends the life of a human being, and it puts women at risk while at the same time isolating them in a time of crisis. The sheer number of abortions included in this study is staggering and heartbreaking. The harm the mothers of these children also experienced is an added tragedy, a tragedy that will continue to unfold as long as the abortion pill remains fully funded by our tax dollars and as long as abortion remains legal.
For more information and background on the abortion pill and the abortion pill rescue, check out our position papers.